2022-02-15 10:16:07
High safety and high environmental adaptability are the basic requirements for the electrolyte of lithium-ion power batteries. With the continuous improvement and update of electrode materials, the requirements for corresponding electrolytes are also stricter. Because it is very difficult to develop a new type of electrolyte system, the common electrolyte of carbonate solvent and lithium hexafluorophosphate is still the main choice of power battery for a long time in the future. In order to meet the development of electrode materials and power batteries for different purposes, a small amount of additives are added to the conventional electrolyte. Anti-overcharge additives, flame retardant additives can make the battery safety improved in the overcharge, short circuit, high temperature, acupuncture and thermal shock and other abuse conditions. Positive film additives can be to meet charge and discharge requirements for high voltage materials in a certain extent.
At present, there are many researches and reports about cyclotriphosphazene compounds, ethoxy-(pentafluoro)-cyclotriphosphazene (PFN) has attracted more attention among these, and it has excellent flame retardant properties with no effect on battery performance in the normal condition [1].
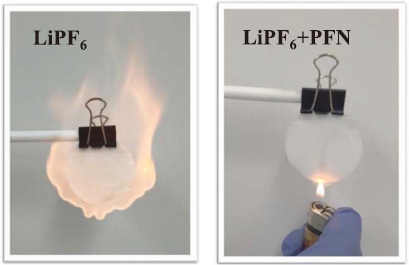
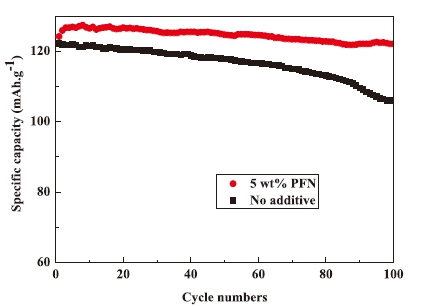
PFN has been widely known as a flame retardant additive for lithium-ion battery, but there are few reports on other functions. Recently, Liu et al. [1] reported that PFN can be used as an electrolyte additive for high voltage lithium batteries. For the LiNi0.5Mn1.5O4 battery, the cycle performance at 0.2C with voltage variation from 3.5V to 4.9V is shown below. It is shown that the capacity retention ratio with PFN addition is superior to the one without PFN.
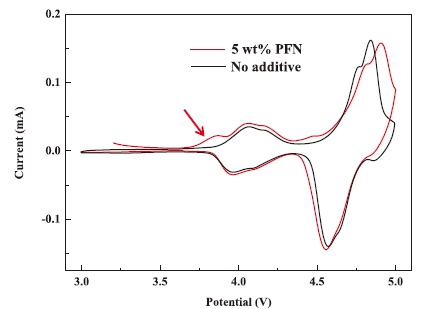
For the LiNi0.5Mn1.5O4 system with PFN and without PFN as additive, cyclic voltammetry experiments show that the system with 5wt% PFN occurs a small oxidation current peak at 3.8V for the first cycle, while this is not the case for the base electrolyte. The results demonstrate that the PFN oxidation occurred, and the oxidation potential of PFN was lower than the decomposition potential of the LiPF6-based electrolyte (4.8 V Li+/Li). The above results indicate that the PFN molecule has been oxidized on the surface of the positive electrode to form a protective layer before the electrolyte is decomposed, and the oxidative decomposition of the electrolyte is suppressed to improve the high-voltage performance of the positive electrode.
In summary, PFN is both as a flame retardant and as an additive for high voltage lithium-ion battery. For PFN as an additive for high voltage, it forms a protective layer on the cathode surface, suppressing electrolyte decomposition and electrode corrosion, and improving the cycle stability of the battery.
References:
[1] J. Liu, X. Song, et al. Nano Energy, 2018, 46, 404-414
Edited by Suzhou Yacoo Science Co., Ltd
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service