2023-09-08
Product Name/English Name:[4-chloro-3-[(3S,5R)-1-chloro-3'-methoxyspiro[adamantane-4,4'-dioxetane]-3'-yl]phenyl] phosphate
English abbreviation:CDP-Star
CAS:160081-62-9
Molecular Formula:C18H19Cl2Na2O7P
Article No.:C0020
Structural Formula:
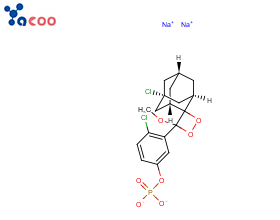
Product Introduction
CDP Star is an ALP chemiluminescent substrate that is commonly used to label biological macromolecules with ALP due to its rapid and high sensitivity characteristics. CDP Star can achieve rapid and ultra sensitive detection of biomolecules by generating extremely fast visible light. In recent years, CDP Star chemiluminescence substrates have been widely used in Western blotting techniques such as Northern blot, Southern blot, and immunoassay.
Application of CDP-Star
AIDS is an immunodeficiency disease caused by HIV infection. At present, there is no effective treatment method or preventive vaccine for this disease. Diagnosis of HIV infection and identification of early infected populations is the primary task of AIDS prevention and control work. Therefore, it is very important to establish sensitive and practical detection methods for detection, diagnosis or blood screening to control the prevalence of AIDS.
There are currently over 100 methods for detecting HIV, with the most commonly used methods for detecting HIV antibodies including ELISA, PA, IFA, and dot blot. ELISA is currently the commonly used primary screening method in clinical practice. However, due to its low sensitivity, it was limited, and later a more sensitive chemiluminescence immunoassay method was developed. However, the chemiluminescence immunoassay kits in existing technologies are all closed and fully automated chemiluminescence measurement systems, requiring expensive fully automated chemiluminescence measurement instruments, which limits their widespread use and cannot be effectively applied in clinical diagnosis and scientific research. Therefore, the CN101178404A patent provides a human immunodeficiency virus antibody chemiluminescence immunoassay diagnostic kit and its preparation method, which includes the following components:
1) Solid phase carriers coated with anti FITC antibodies: microporous plates, plastic beads, plastic tubes, or magnetic particles;
2) FITC labeled HIV recombinant antigen;
3) Enzyme labeled HIV antigen;
4) The chemiluminescent substrates acted on by enzymes include (adamantane) -1,2-dioxoethane, 3- (2 '- spiral adamantane) -4-methoxy-4- (3' - phosphoryloxy) phenyl-1,2-dioxoethane, CSPD or CDP-Star, luminol or isoluminol.
5) Negative control solution: normal human serum.
6) Positive control solution: A positive mixture diluted with newborn bovine serum.
Compared with existing chemiluminescence immunoassay detection methods, this method can improve the sensitivity, homogeneity, and reproducibility of the analysis, and reduce the use of HIV recombinant antigens. This detection method can detect extremely small amounts of viral antibodies in the early stages of antibody production, which can be diagnosed several days earlier than traditional enzyme-linked immunosorbent assay.
References
CN101178404A Human Immunodeficiency Virus Antibody Chemiluminescence Immunoassay Diagnostic Kit and Its Preparation Method.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service