2022-02-15 10:20:01
Good’s buffer is especially dedicated to life science research that does not participate in and does not interfere with biochemical processes. Here we introduce two commonly used Good’s buffers PIPES and HEPES.
PIPES
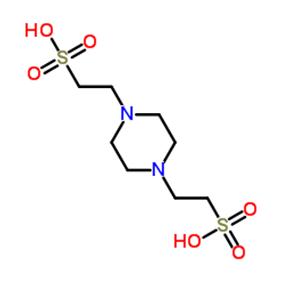
PIPES has a pH buffer range from 6.1 to 7.5, is insoluble in water, but soluble in aqueous NaOH. PIPES differs from buffers containing bis(2-hydroxyethyl)amino groups (such as Bis-tris, Bicine) which does not form stable complexes with most metal ions. It is suitable for buffers in solution systems containing metal ions. According to the existing research results, PIPES can be applied to purify microtubule proteins using phosphocellulose chromatography, to purify recombinant GTP-binding proteins ARF1 and ARF2 by gel filtration, and as a buffer solution to crystallize a transketolase enzyme from E. coli. In addition, PIPES can form radicals and therefore not suitable for redox reactions. In cation exchange chromatography, low concentrations of PIPES buffer should be used because PIPES has a relatively large ionic strength and its pKa value depends on concentration.
HEPES
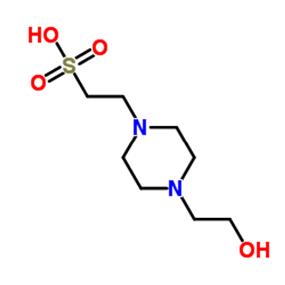
The pH buffer range of HEPES is from 6.8 to 8.2. It is soluble in water and does not form stable complexes with metal ions. In most cases, it will not interfere with biochemical processes. HEPES is commonly used in cell culture media for various types of organisms; PIPES is commonly used as a binding buffer and an eluent during cation exchange chromatography for protein studies; in DNA studies, PIPES is used as a buffer to form precipitates of calcium phosphate and DNA, and as a buffer in AFM and electroporation experiments. In addition, HEPES interferes with the reaction between DNA and restriction enzymes, and is not suitable for use with the Lowry method of protein quantitation.
In summary, both PIPES and HEPES are Good's buffers and cannot form stable complexes with metal ions. They are suitable for solution systems containing metal ions. However, they also have differences in properties. In terms of solubility, PIPES is insoluble in water, while HEPES has good water solubility; in terms of buffer range, PIPES is suitable for acidic and neutral system, and HEPES is suitable for neutral and alkaline system. This is mainly due to the structural difference between the two molecules, PIPES has two sulfonic acid groups, and HEPES contains a sulfonic group and a hydroxyl group. In addition, PIPES and HEPES have some limitations in certain systems. Therefore, it is to consider comprehensively the studied system and the different properties between them.
Edited by Suzhou Yacoo Science Co., Ltd
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service