2018-01-17 17:03:03
Functional electrolyte additives are one of the key materials that affect the electrochemical performance (energy density, power density, cycle performance) of lithium-ion battery. They mainly include film-forming additives, flame retardant additives, overcharge protection additives, high-temperature or low-temperature electrolyte additives, etc. These additives include unsaturated organic compounds, P / N / F / S-containing compounds, new lithium salts and so on. Below we introduce four new lithium salts and the corresponding function.
Lithium bis(oxalate)borate (LiBOB)
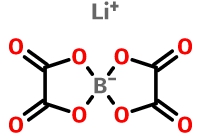
LiBOB is the first nonfluorinated salt, which was reported in a German by Metallgesellschaft AG in 1999 [1]. Later, the researchers found that LiBOB has good high temperature stability (decomposition temperature is 302 °C), the electrolyte with LiBOB has a good high-temperature stability; excellent ability of film formation, making graphite anode stably in PC solution, forming a uniform low impedance SEI film, improve the cycle life of lithium-ion battery. LiBOB as an additive can improve the electrolyte's high temperature and cycle performance.
Lithium bis(fluorosulfonyl)imide (LiFSI)
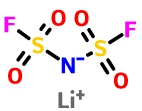
LiFSI has good high temperature stability with thermal decomposition temperature higher than 200 °C; in the presence of a small amount of water will not release HF gas. It was found that high purity LiFSI with carbonate solvent did not corrode aluminum current collectors at a high potential of 3-5 V vs. Li + / Li, forming an aluminum passivation layer at 4.2 V vs. Li + / Li. The use of LiFSI as an addivite improves cycle life, charge/discharge performance, low-temperature performance and cell bulging caused at high tempreture.
Lithium bis(trifluoromethanesulphonyl)imide (LiTFSI)
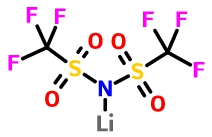
LiTFSI has good thermal stability and chemical stability, decomposition temperature up to 370 °C, has good water stability. Sharova et al. [2] found that the addition of 2wt% LiTFSI in the electrolyte can effectively improve the cycle life of LiFePO4 / graphite cell. This is mainly due to the more LiF in SEI film formed on the surface of graphite cathode which benefits from the addition of LiTFSI. SEI film is thinner and more stable, thereby reducing the decomposition of the electrolyte and reducing the interfacial resistance. The experimental results of Sharova et al. Show that LiTFSI as an additive are more suitable for use at room temperature.
Lithium Difluorophosphate (LiPO2F2)
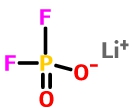
As a phosphorus-containing salt, LiPO2F2 has been found that its combination with VC can improve the rate capability and cycle stability of cell. LiPO2F2 as a single additive can improve the low temperature performance of LiNi0.5Co0.2Mn0.3O2 / graphite cell [3]. In addition, Yang et al. found that LiPO2F2 can form a film on the cathode surface, effectively improving the cycle life of the LiNi0.5Co0.25Mn0.25O2 electrode at room temperature or high temperature [4].
References:
[1] Lischka U , Wietelmann U , Wegner M, Lithium bis(oxalato)borate, method for its production and application, DE19829030C1, 1999.
[2] V. Sharova, A. Moretti, et al. Comparative study of imide-based Li salts as electrolyte additives for Li-ion batteries: Journal of Power sources, 2018, 375, 43-52.
[3] B. Yang, H. Zhang, et al., Lithium difluorophosphate as an additive to improve the low temperature performance of LiNi0.5Co0.2Mn0.3O2/graphite cell: Electrochimica Acta, 2016, 221(10), 107-114.
[4]W. Zhao, Y. Ji, Y. Yang, et al. Recent advances in the research of functional electrolyte additives for lithium-ion batteries, Current Opinion in Electrochemistry, 2017, 6, 84-91.
Edited by Suzhou Yacoo Science Co., Ltd.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service