2017-09-12
Poly(3,4-ethylenedioxythiophene) (PEDOT) is a kind of heterocyclic conductive polymer. Since its first synthesis in the 1990s, its excellent electrochromic properties have become a hotspot in the field of functional materials. In this paper, the principle of electrochromism, the main synthesis methods, and the application of PEDOT are described.
Principle of Electrochromism
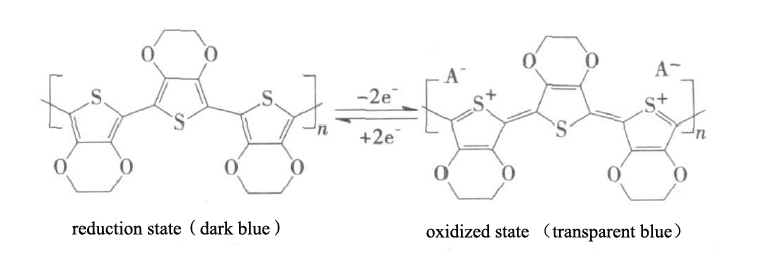
Organic thiophene electrochromic materials have the advantages of high hole mobility, high electrical conductivity, good environmental stability and good film-forming properties, which makes them quickly become the focus of attention. PEDOT has two oxygen atoms directly connected to the thiophene ring, reducing the bandgap width, and it will not produce space reverse to reduce the degree of conjugation due to its structural properties. PEDOT has better redox stability, solubility and conductivity than polythiophene and methyl-substituted thiophene polymers. Under the action of the external voltage signal, the PEDOT undergoes the reversible change of the conjugate structure after the doping of the anion and the Injection and extraction of electrons. It shows the reversible transformation between the transparent blue in the oxidized state and the dark blue in reduction state [1,2], see Figure 1 below:
The main synthesis methods of PEDOT
The synthesis of PEDOT uses polymerization of monomer EDOT: EDOT•+ is formed by the voltage, oxidants from EDOT, two EDOT•+ remove two hydrogen ions to form dimers, the dimer then form cationic radical and coupled with other cationic radicals to grow the chain, resulting in a long chain of PEDOT.
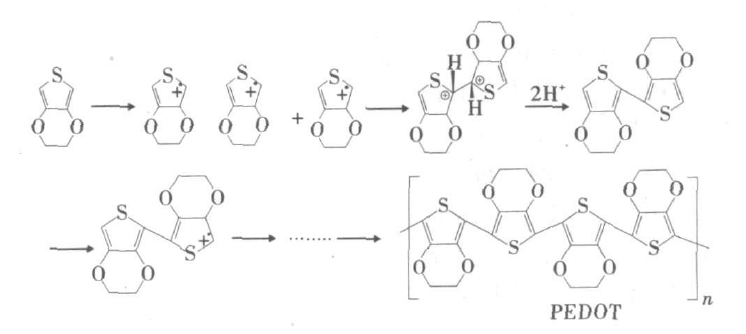
According to the conditions which initiate the formation of cationic radicals from the monomer, the synthesis can be divided into chemical oxidation polymerization and electric polymerization [1].
Chemical oxidative polymerization: EDOT·+ is formed by the trigger of oxidzing agent. After dissolving the polymer in the solvent, it is coated on the conductive substrate by dipping, dropping or spin. Generally, ferric chloride is often used as oxidant, but its oxidation polymerization rate is too fast, it is not easy to control the form of product;
Electropolymerization: PEDOT polymer film was deposited directly on the electrode surface with the electrode force as the initiator and driving force of the polymerization reaction. Although the required equipment is complicated and it is not suitable for preparing film with large quantity and larger area, it can be directly coated on the conductive substrate, and the thickness and the coating rate of the film can be controlled by controlling the polymerization charge and current, etc., so the electropolymerization is the most common method to prepare PEDOT.
Application of PEDOT Electrochromic Material
Electrochromic materials can be applied to intelligent windows, display screen, memory and military camouflage, etc. [2]. PEDOT not only has a short response time, high coloring efficiency, and its environmental stability is also very good, so it have broad application prospects in many areas, such as organic light emitting diodes, super capacitors, optical attenuators and so on. In recent years, PEDOT has also been considered to be the electrochromic self-adaptive camouflage material closest to practical. Its application to military camouflage mainly lies in optical and infrared camouflage.
PEDOT electrochromic materials have the advantages of good environmental stability, easy to synthetise and short response time, and become the focus of conductive polymer research. With the continuous research and development, to improve the service life of materials, increase the richness of color, we believe in the near future, more PEDOT electrochromic materials will be presented in the industrial, life, military and other fields.
References
[1] Tao Yijie, Zheng Wenwei,
Cheng Haifeng, et al. Research progress of electrochromic conductive polymer
PEDOT. Materials Review, 2010, 7, 113-117.
[2] Jin Shan-shan, Chen Hong-shu, Wang Jie-liang, et al. Advances in
polythiophene electrochromic materials. Bulletin of Polymer Science, 2014, 3,
65-74.
Edited by Suzhou Yacoo Science Co., Ltd.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service