How long can eye drops be used once opened?
In many people's views, it can still be used if there is no precipitation and within the expiration date. In fact, it should be discarded immediately if not used up after 4 weeks once opened.
After exposure to air, eye drops would be infected by bacteria, in order to prevent deterioration, bacteriostats polidronium chloride and benzalkonium chloride will be added. But which one is better? Is there any harm to the human body?
Benzalkonium chloride (BAC) is one of the most widely used ophthalmic bacteriostatic agent, and about 70% of the ophthalmic preparations listed in Europe, America and Japan have used BAC as a bacteriostat, its safety and efficacy have been recognized. Polidronium chloride (polyquaternium-1, PQ-1), a new type of antimicrobial agent developed by Alcon in the United States, was used in the early 1990s for contact lenses solutions, then used as antibacterial agent for eye drops. PQ-1 is considered to be a new generation of ophthalmic preparation antibacterial agent with less toxic and better effect.
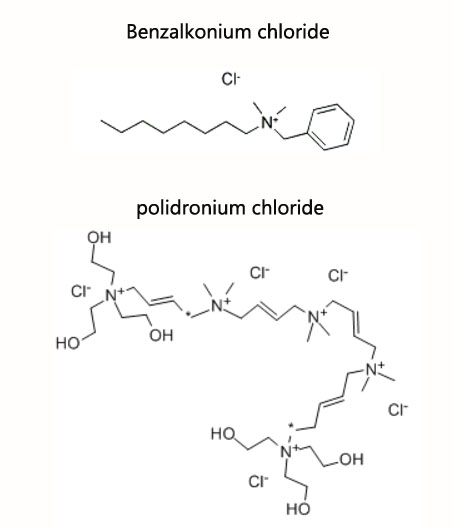
The concentration of BAC in most ophthalmic preparation is 0.004%~0.02%; while the vast amount of PQ-1 (CAS 75345-27-6) is 0.001%. But it is not intuitively believed that the bacteriostatic concentration of PQ-1 is much less than that of BAC, because they are often associated with other adjuvant antibacterial agents in the prescription of ophthalmic preparations.
Some in vitro studies have shown that the cytotoxicity of BAC is significantly correlated with the concentration: the lower the concentration, the lower the toxicity; While the toxicity of PQ-1 to in vitro cultured human corneal epithelial cells and conjunctival epithelial cells is lower than BAC.
But other experiments show that addition of BAC within the limits does not show significant cytotoxicity, the eye adverse reactions caused by BAC were extremely mild and were not significantly higher than those in the control group, and there was no significant difference in clinical safety between BAC and PQ-1 (Model No.: P0142).
From the clinical results of many years, the trace amount of antimicrobial agents in the eye drops have no harm to the eyes. Rational use of ophthalmic preparations will help to release eye adverse reactions and improve tolerance
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service