Buffer solution is usually a solution composed of "weak acid and its conjugate base" or "weak base and its conjugate acid", which can slow down the pH change when a certain amount of other substances are added. Good's buffers are zwitterionic buffers, which are generally sulfamic acid-based organic compounds, their pH values are relatively stable and does not react with various enzymes and chemical reagents. It is mainly used in the field of biology and medicine. CAPS (N-Cyclohexyl-3-Aminopropanesulfonic Acid) is one of the commonly used Good’s buffers, we will mainly introduce it below.
Commonness of Good’s Buffers (including CAPS)
(9) Does not participate in biochemical reactions and does not interfere with biochemical reactions.
Application of CAPS
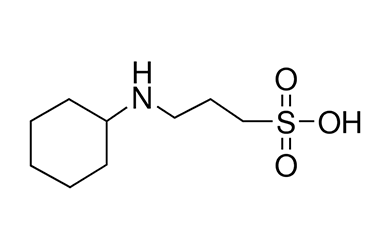
CAPS (CAS 1135-40-6) has a pKa value of 10.4 at 25°C and a pH buffer range of 9.7~11.1. It can be used in the following experiments:
(7) Suitable for the determination of bicinchoninic acid (BCA).
Preparation method for CAPS buffer
Please see the following table (Both concentrations of buffer and sodium hydroxide are 0.1 mol/L):
pH of CAPS Buffer
Volume (CAPS)
Volume (Sodium Hydroxide)
6.8
250
0
10.0
218.3
31.7
10.5
178.6
71.4
10.8
156.3
93.7
11.2
138.9
111.1
The specific operation of the preparation is: 0.1 mol/L buffer solution is first prepared in a volumetric flask. During the configuration process, the buffer has to be dehydrated at a high temperature and cooled in a desiccator; 0.1 mol/L sodium hydroxide solution is also formulated at the same time. According to the formula in the above table, a buffer solution corresponding to pH can be prepared, and sodium hydroxide is mainly used to adjust the pH.
It should be pointed out that most protein experiments should be performed at 4°C to prevent protein degradation. The configured buffer needs to be sealed and stored, used up as soon as possible after opening, and disposable masks and lab coats should be worn during the experiment to ensure the safety and health of the experimenters.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service