(1) The preparation of HEPES
The synthetic route
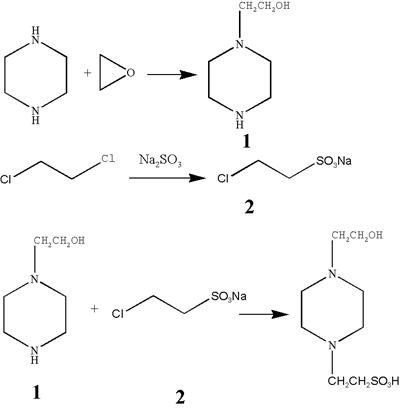
The piperazine hexahydrate was used as a raw material, dissolved in ethanol to form a color transparent solution, then ethylene oxide was added while refluxing at 80°C. Once the reaction is completed, ethanol was distilled off under reduced pressure, and the residue was separated and purified by vacuum fractionation, distillate of 125~127°C was collected (Compound 1).
First, anhydrous sodium sulfite and water were added to the flask, the mixture was heated in a water bath. When the temperature reached 90°C, 1,2-dichloroethane was added dropwise, and the reflux was continued. The reaction liquid was concentrated under reduced pressure in a water bath of 70°C to a white solid, the solid was suspended in 95% ethanol, and the mixture was heated to reflux and filtered to remove insolubles. The supernatant was allowed to stand at 0°C overnight. White flaky crystals were collected by suction filtration using a Buchner funnel.
The compound 1, 2 and water were placed in a flask, stirred to a colorless transparent solution (pH>13), and externally incubated at 110°C~115°C in an oil bath. Stir it for 20 min and the pH of the reaction solution gradually decreased to 9, and 5N sodium hydroxide was added dropwise to maintain the pH of the reaction solution between 9 and 10. When the pH of the reaction solution is not decreasing, sodium hydroxide is stopped and reflux is continued until the reaction is completed.
The cooled reaction solution was then diluted with water and purified by ion-exchange resin [H+]* column. The collected solution obtained by chromatography was concentrated under reduced pressure, and then filtered and evaporated. The colorless transparent clear solution was further concentrated to a thick syrup, poured into boiling 95% ethanol, adjusted to pH 5 with glacial acetic acid, and immediately add absolute ethanol to precipitate white crystals, naturally cooled at room temperature, and place at 0°C for overnight.
The crystals were collected by suction filtration using a Buchner funnel and washed with ice cold ethanol. The washed crystals were suspended in 95% ethanol, heated to 75°C, and water was added dropwise to dissolve them completely. After hot filtration, the supernatant is added with absolute ethanol, and naturally cooled under shaking to precipitate the white crystals in a loose form. Leave at 0°C overnight.
The crystals were collected by suction filtration using a Buchner funnel, washed with ice-cold anhydrous ethanol, and dried at 80°C to give the product.
(2) Preparation of HEPES buffer
Depending on the application, HEPES + NaOH and HEPES + salt are commonly used:
According to the report of Lepe-Zuniga et al., HEPES has a phototoxicity when exposed to ambient light by the production of hydrogen peroxide. It is thus strongly advised to keep HEPES-containing solutions in darkness as much as possible.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service