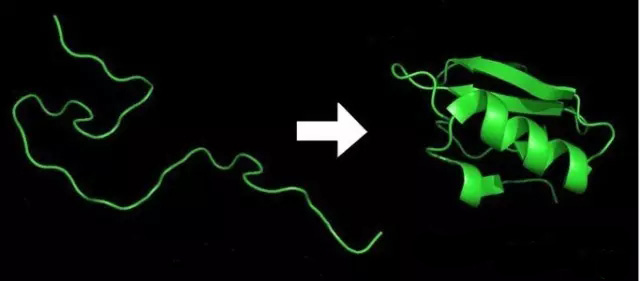
With the development of genetic recombination technology, more and more proteins can be expressed in exogenous host cells. However, in the process of expression, incorrect folding leads to aggregation of protein to form inclusion body. On the one hand, we can avoid the formation of inclusion body by changing the culture conditions of upstream cells, such as lowering the expression temperature, reducing the culture time, and decreasing the concentration of the inducer IPTG. However, if the situation cannot be changed, the inclusion body must be purified. And guanidine hydrochloride plays a huge role in the purification of inclusion body.
(1) Breaking Cells
The bacteria were collected for centrifugation to obtain crude inclusion bodies.
(2) Washing of Inclusion Bodies
The inclusion bodies are washed with a buffer containing low concentration of denaturant (such as 2M urea) or no denaturing agent, to remove a part of the heteroprotein, and the purified inclusion body is obtained by centrifugation.
(3) Dissolution of Inclusion Bodies
The purified inclusion bodies are solubilized using buffer containing high concentration of denaturant, such as 8M urea or 6M guanidine hydrochloride.
Urea or guanidine hydrochloride (CAS 50-01-1) have different abilities to dissolve inclusion bodies. Urea and guanidine hydrochloride are moderate intensity denaturing agents, which have strong reversible denaturing effects on hydrogen bonding of inclusion bodies, but the ability of urea is slower and weaker than that of guanidine hydrochloride. The required concentration of urea is usually 8M, which has a poor dissolution effect on about one-third of the protein inclusion bodies, and the solubility is 70%~90%; while the concentration of guanidine hydrochloride is usually 6M, and it can dissolve most inclusion bodies, the dissolution capacity is over 95%.
(4) Renaturation and Purification
The dissolved inclusion bodies can be renatured by dilution or dialysis before being purified; or purified and then renatured. Alternatively, the dissolved inclusion body protein is subjected to on-column refolding to achieve renaturation and purification simultaneously. In general, molecular sieve (SEC), metal ion chelate chromatography (Ni2+), ion exchange (IEX) and hydrophobic (HIC) can tolerate high concentrations of denaturant, so different chromatographic techniques can be used for on-column refolding. However, it is necessary to pay attention to whether the high concentration of the denaturant or other components in the buffer will affect the interaction between the protein and the filler.
(5) Detection and analysis
Finally, the obtained sample is subjected to detection and analysis to see if a natural protein is obtained. General detection methods include biological activity detection, circular dichroism, dynamic light scattering or gel filtration, reversed phase chromatography, and so on.
Inclusion body renaturation is a very complicated process and is subject to many factors. The concentration of various reagents and proteins in the experiment, the operating temperature and renaturation time, the composition of the buffer and the pH value are all crucial to the experimental results. Although Guanidine hydrochloride has shortcomings like high cost, precipitation under acidic conditions, interference with protein ion exchange chromatography after renaturation in the process of dissolving inclusion bodies, its solubility is strong and does not cause covalent modification of recombinant protein.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service