2024-03-19
Product Name:MOPSO
CAS: 68399-77-9
Molecular Formula:C7H15NO5S
Article No.: M0003
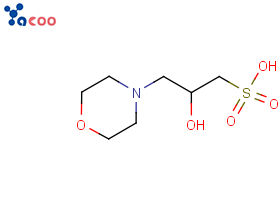
Structural Formula:
Product Introduction
Mopso is a zwitterionic bio buffering reagent with a structure similar to MOPS and an effective pH range of 6.2-7.6. MOPSO is mainly used as a biological buffer for biochemical research.
Application of MOPSO
Human chorionic gonadotropin (HCG) is a glycoprotein secreted by the trophoblast cells of the placenta α and β The glycoprotein composition of dimers. The main function of HCG is to stimulate the corpus luteum, which is beneficial for the continuous secretion of estrogen and progesterone to promote the formation of the uterine decidua and promote the growth and maturation of the placenta. HCG proliferates rapidly in the first 8 weeks of pregnancy to maintain pregnancy. After approximately 8 weeks of pregnancy, HCG gradually decreases and reaches relative stability around 20 weeks. The use of HCG dual antibodies to detect the HCG content in the urine of pregnant women can quickly determine the results in the early stages of pregnancy, and is an effective means of auxiliary diagnosis. Research has shown that pregnant women not only contain HCG in their urine, but also in their saliva. The comparison of the results of the two samples shows a good correlation and consistent examination results. Saliva testing for early pregnancy has many advantages, such as being cleaner, more convenient, tested at any time, and not affected by sample collection time. However, saliva testing strips for early pregnancy are not often mentioned, and the development difficulty lies in the presence of many non detection interferents, low sensitivity, difficult chromatography, and non-specific issues in saliva samples. Therefore, the CN117129691A patent developed a test strip for detecting human chorionic gonadotropin in saliva. The test strip consists of 5 parts: sample pad, bonding pad, water filter pad, coating film, and bottom plate.
Among them. The combination pad treatment solution consists of 4 parts: 3- (N-morpholino) -2-hydroxypropanesulfonic acid, casein, Tween-20, and water. The specific steps are: Weigh 1.13g of 3- (N-morpholino) -2-hydroxypropanesulfonic acid, 0.5g of casein, Tween-2050 μ Put L into a small beaker containing 80mL of pure water, stir and dissolve, adjust the pH to 8.5, and finally make up to 100mL for use.
The test strip prepared by the method of this patent is clean and convenient for detecting early pregnancy, with high sensitivity and strong specificity.
References
CN117129691A A test strip for detecting human chorionic gonadotropin in saliva and its preparation method.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service