2017-09-01
Saxagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor, a drug developed by Bristol-Myers Squibb Pharmaceuticals Inc. for the treatment of adult type II diabetes, which was approved by the FDA in March 2010, and by the SFDA in May 2011. It has the advantages of prominent and lasting efficacy. This paper will summarize and compare the main synthetic routes.
Route 1: WO2004052850 [1]
3-hydroxyadamantane-S-glycine is reacted with Boc2O under the presence of sodium hydroxide to give 3-hydroxyadamantane-Boc-S-glycine, which then reacted with (1S,3S,5S)-2-azabicyclo[3.1.0]hexane-3-carboxamide, the formyl group in the condensation product is dehydrated into cyano group by reaction with trifluoroacetic anhydride, then the product is deprotected to give the target compound.
Route 2: WO2005094323 [2]
The route is different from the route one in that the acid chloride is used to carry out the peptide coupling reaction, and the nitrogen-terminated trifluoroacetyl protecting group is removed by sodium borohydride.
Route 3: J. Med. Chem. (2005, 48, 5025-5037) [3]
The 3-hydroxyadamantane-Boc-S-glycine was first prepared as an active ester and then reacted with (1S,3S,5S)-2-azabicyclo[3.1.0]hexane-3-carboxamide, dehydrated by trifluoroacetic anhydride, and removed the protective group to get the target product.
Route 4: CN105503698A [4]
3-hydroxyadamantane-Boc-S-glycine was reacted with (1S,3S,5S)-2-azabicyclo[3.1.0]hexane-3-carboxamide under the presence of organic base and 1-Propanephosphonic acid cyclic anhydride (T3P). The product 4 was dehydrated under the presence of T3P to give the product 5, which was deprotected to give the target compound.
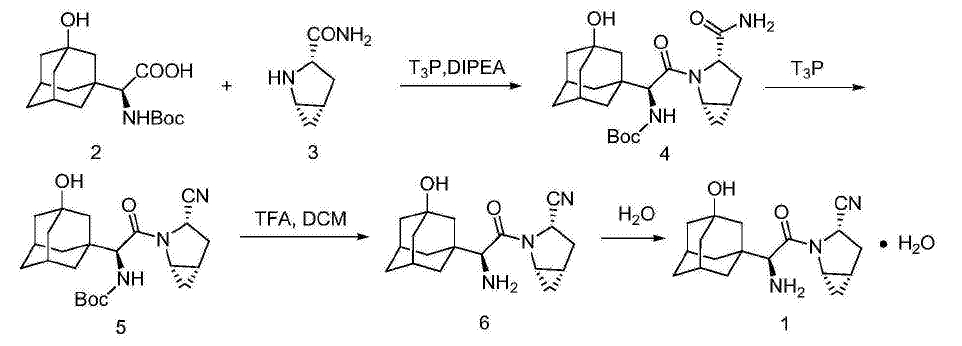
In the first three routes, the relevant intermediate are obtained by turnning 3-hydroxyadamantane-Boc-S-glycine into mixed anhydride, acid chloride or active ester, then reacting with the corresponding amine. The reaction conditions of the mixed acid anhydride are relatively mild, but methanesulfonyl chloride has some danger in the process of use, there may be some problems in the productive process. 3-hydroxyadamantane-S-glycine and trifluoroacetic anhydride need a long time for reaction in Route 2, which resulting in a significant proportion of racemization, the purity of the target product is not high; the reaction conversion rate of activated ester in Route 3 is not high, which affects the purity of the product.
In comparison with the above routes, Route 4 uses T3P for the peptide coupling reaction and the dehydration reaction of formamide, and preferably uses ethylene glycol diethyl ether as the solvent in the formamide dehydration, which increase the reaction temperature in the step of dehydration, shorten the reaction time, reduce the occurrence of racemization, improve the purity of the product, and reduce the reprocessing toxicity.
References
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service