2017-09-04
Recently, Daiichi Sankyo's official website has published an important outcome of a Phase III clinical trial of its drug Mirogabalin: Mirogabalin has a significant effect in the treatment of diabetic peripheral neuropathy (DPN) and has no major safety concerns.
Diabetes is an important public health problem, according to the data from World Health Organization, there are about 400 million people around the world. Diabetes can affect other parts of the body, causing a variety of complications. DPN is one of the most common long-term complications of diabetes, the incidence rate is up to 90%. The early symptoms are mainly sensory disorder, when the motor nerve is involved later, there may be muscle atrophy, myasthenia and so on. Studies have confirmed that factors associated with type 1 diabetes, type 2 diabetes, and both of them are all responsible for DNA damage, endoplasmic reticulum stress, mitochondrial dysfunction, and irreversible damage to the cells, then leading to diabetic neuropathy.
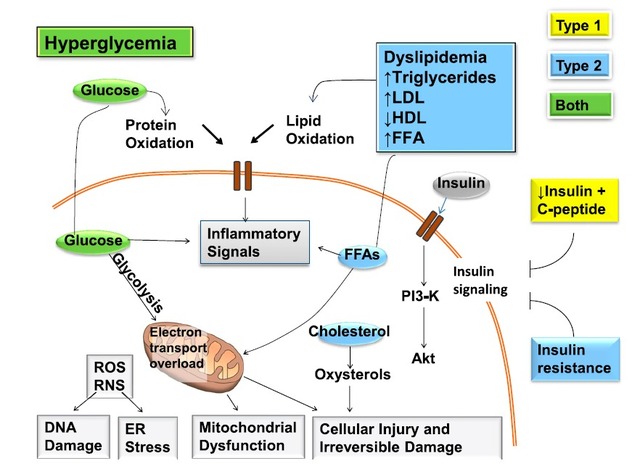
Although there are more than a dozen hypoglycemic agents, including sulfonylurea drugs, such as glibenclamide, glimepiride; thiazolidinedione drugs such as pioglitazone and rosiglitazone; SGLT-2 inhibitors such as Canagliflozin; DPP-4 inhibitors such as Sitagliptin or vildagliptin (intermediate 3-amino-1-adamantanol); biguanides such as metformin, diabetic peripheral neuropathy is usually not adequately reported, the treatment is also more difficult.
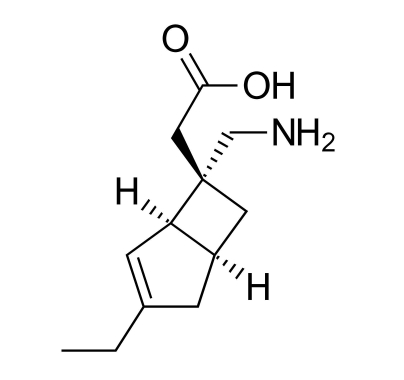
The company is dedicated to the creation and supply of innovative drugs to meet the unsatisfied medical needs of patients. Mirogabalin is an oral therapeutic regimen for pain in the nervous system that preferentially binds to the α2δ-1 subunit of the calcium channel, which is widely present in the nervous system-mediated region of pain transmission and modification. Mirogabalin has a unique binding profile and long duration of action.
REDUCER is an Asian, phase 3, multicenter, randomized, double-blind, placebo-controlled 14-week clinical trial of mirogabalin involving 750 patients aged 20 years or older with diabetic peripheral neuropathic pain. The primary goal was to assess the efficacy of Mirogabalin by comparing the average daily pain score (ADPS) from baseline to week 14. The results showed that Mirogabalin significantly reduced ADPS from baseline to week 14 compared with placebo, and did not have significant safety concerns.
The clinical trial provides important clinical data for Mirogabalin in diabetic peripheral neuropathic pain, supporting its potential application in specific pain syndromes, may help diabetic patients stay away from pain. But what need to be reminded is that the the key to treatment of diabetic peripheral neuropathy is to control the blood sugar, only the steady blood glucose can effectively slow down the surrounding blood vessels neuropathy.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service