2017-10-17
3,4-ethylenedioxythiophene (EDOT) is not only a stable conductive compound monomer, but also the basic material of conductive skeleton. The polymer PEDOT has the advantages of low energy gap, low electrochemical doping potential, short response time, high color contrast, good stability and so on. It is widely used in various industrial fields. With the deepening of the application of PEDOT, the synthesis of EDOT is also promoted. This paper will summarize the synthesis of EDOT.
Five-step synthesis
(5) adding quinoline (or other solvents) and copper (or other catalysts) for decarboxylation reaction, the final product EDOT is obtained. The synthetic route is shown in the following figure [1]:
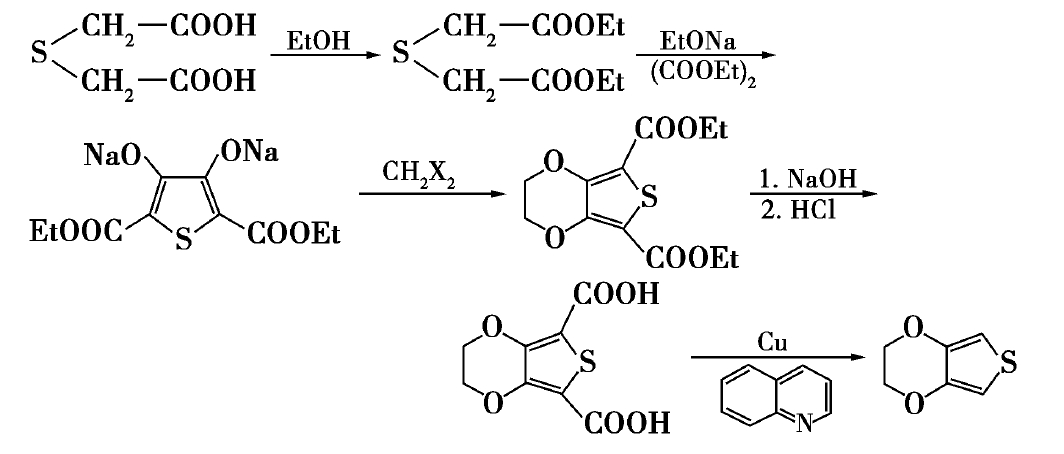
The synthesis process is mature, but the reaction route is too long, resulting in the problem of low yield of final product EDOT, the intermediate purification separation is more cumbersome and so on. In addition, raw materials thioglycolic acid is not easy to buy. Therefore, the researchers have improved the process, using ethyl chloroacetate as raw material, after Claisen ester condensation, acyl chloride, and reaction with chitosan amidation, then occurs O-alkylation reaction with 1,2-dibromoethane in DMF, EDOT was obtained by acid hydrolysis and decarboxylation reaction. The entire synthesis process takes a short time, does not require the use of phase transfer catalyst, the reaction is in the homogeneous system, and the yield is high.
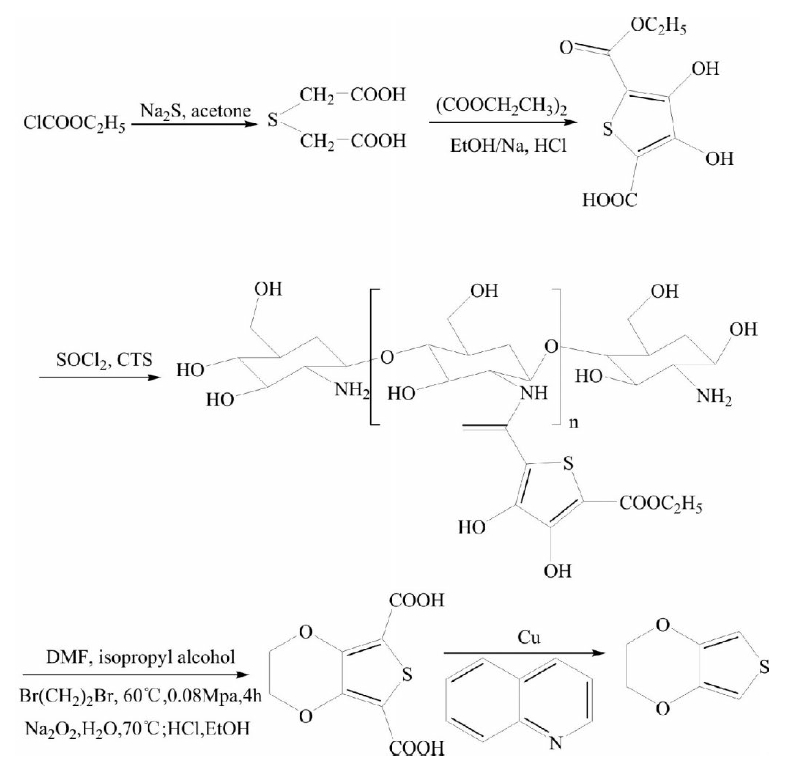
Four-step synthesis
(4) EDOT was obtained by trans-etherification of 3,4-dimethoxy thiophene. The synthetic route is shown below [3]:
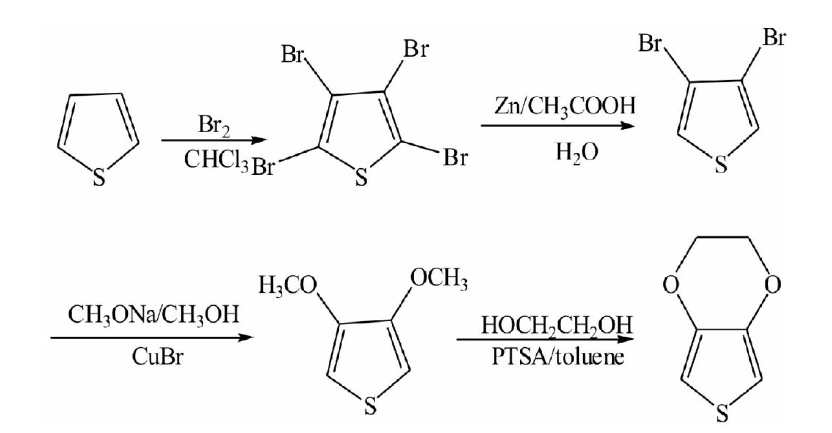
Reaction efficiency of this route is low, and it will produce a waste of reagents, the process will produce toxic hydrogen bromide gas, and need a lot of lye to deal with, post-processing process is complex.
Two-step synthesis
(2) then 3,4-dimethoxy thiophene reacted with ethylene glycol in toluene to produce EDOT, the total yield is 45%.
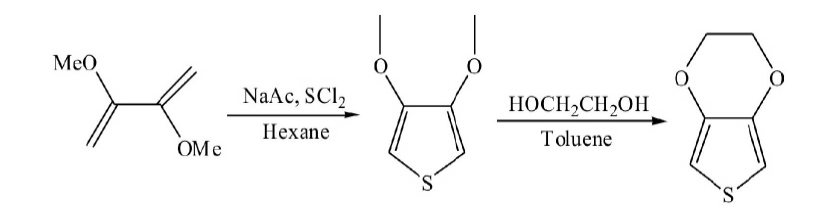
From the perspective of industrial production, this method is more efficient, but the first reaction is cumbersome, and has side effects, and the yield is low. Liu Xiangjie et al. [4] made some improvement on this method, using 3,4-dibromothiophene and sodium methoxide to produce 3,4-dimethoxy thiophene. And the chemical reagents used in the reaction are stable, and the process does not need special operation, the byproduct is relatively low, the yield is relatively high (45%).
One-step synthesis
Using tetrabutylammonium iodide as catalyst and n-hexane as solvent, 2,3-dimethoxy-1,3-butadiene derivative as the raw material, a series of 3,4-ethylenedioxythiophene compounds [5] are produced by macrowave radiation one pot reaction. It has the advantages of simple operation, mild condition, high yield and environment friendliness.
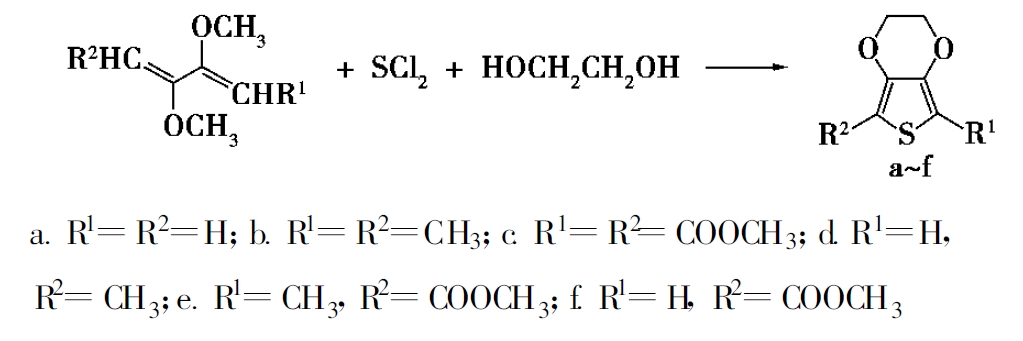
Due to the special structure and property of PEDOT and its derivatives, as well as the continuous improvement of physical modification technology and chemical modification, making the rapid development of conductive polymers, its application areas have also been widened and updated. Synthesis of PEDOT requires a large amount of monomer EDOT, resulting in increasing demand for monomer EDOT, the product quality requirements are getting higher and higher, the synthesis process also need to continue to optimize to meet the green chemistry of industrial needs.
References
[5] Wang Shengwen. Synthesis of 3,4-ethylenedioxythiophenes with microwave irradiation under of one-pot reaction. Chemical reagents, 2009, 31, 1034-1036.
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service