2017-10-16

2017-10-13
2022-02-15

2022-02-15
2022-02-15

2017-09-20
2017-09-18
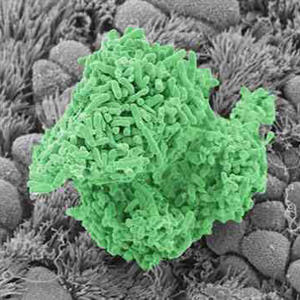
2017-09-15

2017-09-13
Copyright © Suzhou Yacoo Science Co., Ltd. All Rights Reserved
Friendly Links :
online service